
Chemistry, 10.10.2019 02:00 thetudent41
Calculate the number of moles of al2o3 that are produced when 0.60 mol of fe is produced in the following reaction: 2al(s)+3feo(s) = 3fe(s)+al2o3(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Calculate the number of moles of al2o3 that are produced when 0.60 mol of fe is produced in the foll...
Questions



Physics, 19.09.2019 20:00




History, 19.09.2019 20:00


History, 19.09.2019 20:00




Mathematics, 19.09.2019 20:00





Mathematics, 19.09.2019 20:00

Health, 19.09.2019 20:00

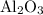 produced is
produced is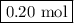 .
.
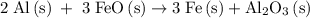
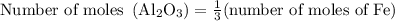 …… (1)
…… (1)
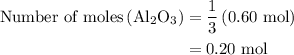
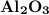 is produced when 0.60 mol of Fe is produced in the reaction.
is produced when 0.60 mol of Fe is produced in the reaction.

