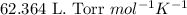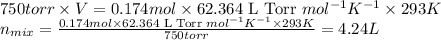Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of a powder. when the mixture is poured down a clogged drain, the following redox reactions occurs: 2naoh(aq) + 2 al(s) + 6h2o(l) → 2naal(oh)4(aq) + 3 h2(g) the heat generated in this reaction melt away grese and the hydrogen gas released stirs up the solids clagging the drain. calculate the volume hydrogen gas formed at 20. ºc and 750. torr if 3.12 g of al is treated with excess naoh.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of...
Questions

Geography, 06.04.2021 22:20


Biology, 06.04.2021 22:20

Mathematics, 06.04.2021 22:20



History, 06.04.2021 22:20

Mathematics, 06.04.2021 22:30





Mathematics, 06.04.2021 22:30



Mathematics, 06.04.2021 22:30


History, 06.04.2021 22:30

Chemistry, 06.04.2021 22:30

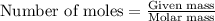
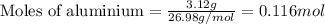
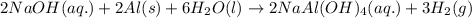
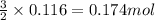 of hydrogen gas
of hydrogen gas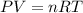
![20^oC=[20+273]K=293K](/tpl/images/0308/3868/3b5d4.png)