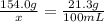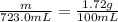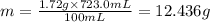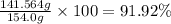The solubility of solid w in water is: 1.72 g/100 ml at 0°c, 21.3/100 ml at 100°c. a) how many ml of boiling water are required to dissolve 154.0 g of w? (report to the nearest ml) if solution were cooled to 0°c, how many grams of w would crystallize out? (report to one decimal place) b) what is the percent recovery? (report to one decimal place) (show calculations)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
The solubility of solid w in water is: 1.72 g/100 ml at 0°c, 21.3/100 ml at 100°c. a) how many ml o...
Questions





Arts, 04.05.2021 20:40

Mathematics, 04.05.2021 20:40



Spanish, 04.05.2021 20:40

Arts, 04.05.2021 20:40



Social Studies, 04.05.2021 20:40

Spanish, 04.05.2021 20:40


Physics, 04.05.2021 20:40

History, 04.05.2021 20:40

Health, 04.05.2021 20:40

History, 04.05.2021 20:40

Mathematics, 04.05.2021 20:40