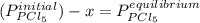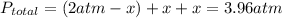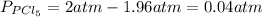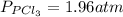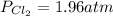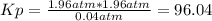Chemistry, 10.10.2019 18:10 lailabirdiemae
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium according to the reaction: pcl5(g) ⇄ pcl3(g) + cl2(g) analysis shows the total pressure in the flask at equilibrium is 3.96 atm. calculate the equilibrium constant kp for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium acc...
Questions

Chemistry, 23.07.2019 04:00



History, 23.07.2019 04:00






History, 23.07.2019 04:00





Mathematics, 23.07.2019 04:00





Biology, 23.07.2019 04:00

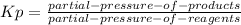
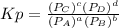
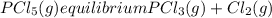
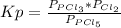
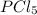 so the total pressure for the system is also the partial pressure for
so the total pressure for the system is also the partial pressure for 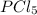 ,
,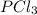 and
and 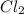 so now we need to estimate the partial pressure for each specie.
so now we need to estimate the partial pressure for each specie. 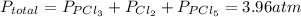
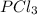 and
and 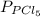 in the equilibrium will be
in the equilibrium will be