
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
e5 = -7 x 10-19 j
e4 = -11 x 10-19 j
e3 = -15 x 10-19 j
e2 = -17 x 10-19 j
e1 = -20 x 10-19 j
(this is not an actual atom that exists. according to the bohr model, energies should follow the expression discussed in section 6.2 of silberberg. this exercise prepares you for calculations like questions 6 and 7 of problem set 1.)
(a) if the electron is initially in the n = 4 level, what is the shortest wavelength of radiation that could be emitted? provide your answer to one significant figure: -7 m.
(b) what is the ionization energy (in kj/mol) of the atom in its first excited state? provide your answer to two significant figures: 103 kj/mol

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

You know the right answer?
Ahypothetical one-electron atom has these energy levels:
e6 = -2 x 10-19 j
...
e6 = -2 x 10-19 j
...
Questions

Mathematics, 09.12.2020 07:50


Mathematics, 09.12.2020 07:50

Advanced Placement (AP), 09.12.2020 07:50

Chemistry, 09.12.2020 07:50

Biology, 09.12.2020 07:50


Mathematics, 09.12.2020 07:50

Mathematics, 09.12.2020 07:50

Mathematics, 09.12.2020 07:50


Arts, 09.12.2020 07:50




Mathematics, 09.12.2020 07:50

Chemistry, 09.12.2020 07:50

Mathematics, 09.12.2020 07:50


Computers and Technology, 09.12.2020 07:50

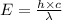
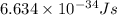
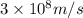
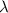 = Wavelength of the radiation
= Wavelength of the radiation
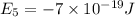
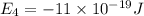
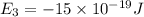
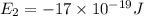
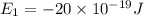

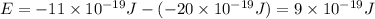
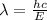

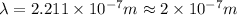
 is the shortest wavelength of radiation that could be emitted.
is the shortest wavelength of radiation that could be emitted.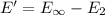
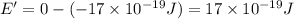
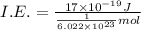
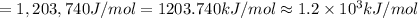
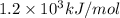 is the ionization energy of the atom in its first excited state.
is the ionization energy of the atom in its first excited state.

