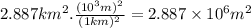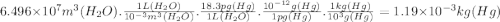Chemistry, 10.10.2019 04:30 carsonspitzer1p5p1g2
An abondoned stone quarry has filled with water to an average depth of 22.50 meters and a surface are of 2.887 km^2. in your lab you analyze five water samples from the quarry and conclude that each liter of quarry water contains 18.3pg of mercury on average.
(a.) what is the volume (in m^3) of the water in the quarry?
(b.) what is the total mass of mercury (in kg) in the quarry?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
An abondoned stone quarry has filled with water to an average depth of 22.50 meters and a surface ar...
Questions






Business, 29.06.2019 08:30





Mathematics, 29.06.2019 08:30


Geography, 29.06.2019 08:40





Physics, 29.06.2019 08:40