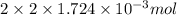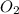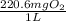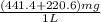Chemistry, 10.10.2019 05:00 geunagillis1
Determine the carbonaceous and nitrogenous oxygen demand in mg/l for a 1 l solution containing 300 mg of a wastewater represented by the formula cn2h602 (n is converted to nh3 in the first step) afterwards calculate the cod of the solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
You know the right answer?
Determine the carbonaceous and nitrogenous oxygen demand in mg/l for a 1 l solution containing 300 m...
Questions

Health, 28.08.2019 19:30

English, 28.08.2019 19:30

Mathematics, 28.08.2019 19:30

History, 28.08.2019 19:30

Chemistry, 28.08.2019 19:30




Mathematics, 28.08.2019 19:30



Biology, 28.08.2019 19:30

History, 28.08.2019 19:30


Health, 28.08.2019 19:30

Chemistry, 28.08.2019 19:30

Computers and Technology, 28.08.2019 19:30

English, 28.08.2019 19:30


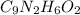 .
.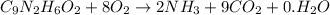
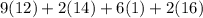
 mg/mol (as 1 g = 1000 mg)
mg/mol (as 1 g = 1000 mg)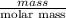
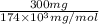
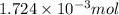
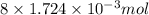
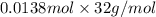
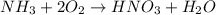
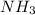 to convert into
to convert into 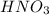 , oxygen required is 2 mol.
, oxygen required is 2 mol.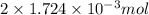 of
of