
Chemistry, 10.10.2019 05:00 akaeiraspruell
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to carbonate (co32-) to determine: hco3 = co2 + h+ k = 10-10.33 (a) whether hco3 or co32- would dominate at ph 9.1 and (b) what the concentration of [co32-] would be at this ph if [hco3 ] = 10-6 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to...
Questions


Mathematics, 28.09.2021 05:40


Mathematics, 28.09.2021 05:50

History, 28.09.2021 06:10

Mathematics, 28.09.2021 06:10

History, 28.09.2021 06:20


Mathematics, 28.09.2021 06:20

Chemistry, 28.09.2021 06:20

Mathematics, 28.09.2021 06:20

Mathematics, 28.09.2021 06:20

Mathematics, 28.09.2021 06:20


French, 28.09.2021 06:30



Mathematics, 28.09.2021 06:30


Computers and Technology, 28.09.2021 06:30

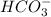 will dominate at pH = 9.1
will dominate at pH = 9.1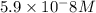
![pH=-\log[H^+]](/tpl/images/0305/9437/cf945.png) ......(1)
......(1)![9.1=-\log[H^+]](/tpl/images/0305/9437/e5ddb.png)
![[H^+]=10^{-9.1}](/tpl/images/0305/9437/b7f9d.png)
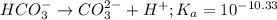
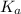 for above reaction follows:
for above reaction follows:![K_a=\frac{[CO_3^{2-}]\times [H^+]}{[HCO_3^-]}](/tpl/images/0305/9437/fea1b.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{[HCO_3^-]}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=\frac{10^{-9.1}}{10^{-10.33}}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=16.98](/tpl/images/0305/9437/786f2.png)
![[HCO_3^-]=16.98\times [CO_3^{2-}]](/tpl/images/0305/9437/e6ce5.png)
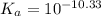
![[HCO_3^-]=10^{-6}M](/tpl/images/0305/9437/1335d.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{10^{-6}}](/tpl/images/0305/9437/27401.png)
![[CO_3^{2-}]=\frac{10^{-6}\times 10^{-10.33}}{10^{-9.1}}=5.9\times 10^-8}M](/tpl/images/0305/9437/65347.png)


