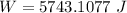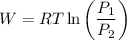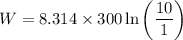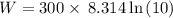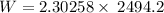Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Calculate the work done when an ideal gas expands isothermally and reversibly in a piston and cylind...
Questions

Mathematics, 21.02.2022 22:20




Mathematics, 21.02.2022 22:30


Mathematics, 21.02.2022 22:30

Health, 21.02.2022 22:30




Arts, 21.02.2022 22:30

Mathematics, 21.02.2022 22:30

History, 21.02.2022 22:30

Mathematics, 21.02.2022 22:30


Mathematics, 21.02.2022 22:30


Mathematics, 21.02.2022 22:40

Social Studies, 21.02.2022 22:40