
Chemistry, 10.10.2019 04:10 princess42044
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2. he pipets 20 ml of this solution into a 100 ml volumetric flask and dilutes to the mark. he then pipets 10 ml of this diluted solution into a 100 ml volumetric flask and dilutes to the mark. he analyzes some of the solution from the final volumetric flask and finds that the iodide ion concentration is 0.574 m. (note: in solution, calcium iodide breaks apart into one ca 2+ ion for every two i - ions, so a solution that is 1.0 m in cai 2 is 2.0 m in i determine the molar concentration of calcium iodide in the original solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

You know the right answer?
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2...
Questions


Mathematics, 27.10.2020 04:00

Mathematics, 27.10.2020 04:00

Mathematics, 27.10.2020 04:00






Mathematics, 27.10.2020 04:00

English, 27.10.2020 04:00


Arts, 27.10.2020 04:00

Computers and Technology, 27.10.2020 04:00


Biology, 27.10.2020 04:00


Mathematics, 27.10.2020 04:00


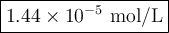
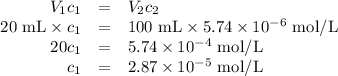
![[\text{Ca}^{2+}] =\dfrac{2.87 \times 10^{-5} \text{ mol I}^{-}}{\text{1 L}} \times \dfrac{\text{1 mol Ca}^{2+} }{\text{2 mol I}^{-}} = \mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}\\\\\text{[Ca$^{2+}$] in the original solution was $\large \boxed{\mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}}$}](/tpl/images/0305/8108/dfb7a.png)


