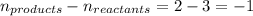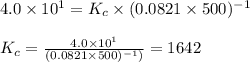The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three gases has partial pressures of 9.10×10−2 atm , 0.174 atm , and 0.24 atm for no, cl2, and nocl, respectively. part apart complete calculate kp for this reaction at 500.0 k. express your answer using two significant figures. kp = 40 previous answers correct part b if the vessel has a volume of 6.00 l , calculate kc at this temperature. express your answer using two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
The equilibrium 2no(g)+cl2(g)⇌2nocl(g) is established at 500 k. an equilibrium mixture of the three...
Questions

Mathematics, 19.08.2021 01:00

Biology, 19.08.2021 01:00



Mathematics, 19.08.2021 01:00

Social Studies, 19.08.2021 01:00


Mathematics, 19.08.2021 01:00

Mathematics, 19.08.2021 01:00



English, 19.08.2021 01:00



Mathematics, 19.08.2021 01:00





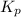 for the given reaction is
for the given reaction is 
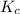 for the given reaction is 1642.
for the given reaction is 1642.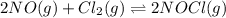
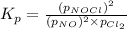
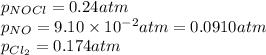
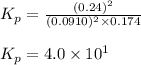
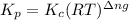
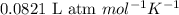
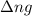 = change in number of moles of gas particles =
= change in number of moles of gas particles =