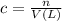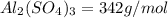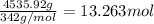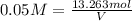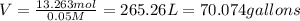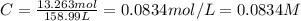Chemistry, 10.10.2019 03:20 Mrdwarf7163
You have an-empty 42-gatbarrel-and-a 10-lb bag of dry alum, al2(so4). (a) calculate the number of gallons of 0.05 m solution you can make with this amount of alumthe-materials you hve on hand. (b) suppose you put all the alum into a 42-gal-the barrel and filled it full calculate the molar concentration of the resulting solution

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

You know the right answer?
You have an-empty 42-gatbarrel-and-a 10-lb bag of dry alum, al2(so4). (a) calculate the number of ga...
Questions

Mathematics, 06.01.2021 18:00


English, 06.01.2021 18:00


Chemistry, 06.01.2021 18:00

Mathematics, 06.01.2021 18:00

Mathematics, 06.01.2021 18:00





Chemistry, 06.01.2021 18:00

Advanced Placement (AP), 06.01.2021 18:00



Social Studies, 06.01.2021 18:00