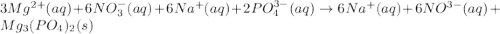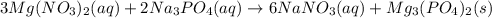When a solution of magnesium nitrate is mixed with a solution of sodium phosphate, the solid that forms is magnesium phosphate. select the equation that represents the complete ionic equation. a. mg(no3)2(aq) + na2po4(aq) mgpo4(s) + nano3(aq) b. mg2+(aq) + po43-(aq) mgpo4(s) c. 3mg(no3)2(aq) + 2na3po4(aq) mg3(po4)2(s) + 6nano3(aq) d. 3mg2+(aq) + 2po43-(aq) mg3(po4)2(s) e. 3mg2+(aq)+6no3-(aq) + 6na+(aq) + 2po43-(aq) mg3(po4)2(s)+6no3-(aq)+ 6na+(aq)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
When a solution of magnesium nitrate is mixed with a solution of sodium phosphate, the solid that fo...
Questions

History, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00


Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00



Chemistry, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

History, 18.12.2020 01:00

Biology, 18.12.2020 01:00


English, 18.12.2020 01:00

Chemistry, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00



Mathematics, 18.12.2020 01:00