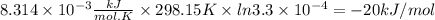Chemistry, 10.10.2019 00:30 idontknow1993
Acetylsalicylic acid (hc9h7o4) is a weak acid that dissociates in water in the following manner. hc9h7o4(aq) + h2o(l) equilibrium reaction arrow c9h7o4−(aq) + h3o+(aq) at a temperature of 298.15 k, the acid-dissociation constant (ka) for acetylsalicylic acid is 3.3 ✕ 10−4. (a) calculate the change in standard free energy (δg°) for this equilibrium reaction. kj/mol (b) what is the value of δg at equilibrium? δg > 0 δg = 0 δg < 0 (c) if the reaction were spontaneous in the forward direction, what type of value would you expect for δg?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
Acetylsalicylic acid (hc9h7o4) is a weak acid that dissociates in water in the following manner. hc9...
Questions


Biology, 18.09.2019 10:00




Health, 18.09.2019 10:00

Mathematics, 18.09.2019 10:00



History, 18.09.2019 10:00


Geography, 18.09.2019 10:00


History, 18.09.2019 10:00


Mathematics, 18.09.2019 10:00



Mathematics, 18.09.2019 10:00

Mathematics, 18.09.2019 10:00