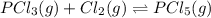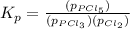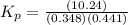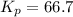Chemistry, 10.10.2019 00:10 GingerSnaps
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: pcl3 (g) + cl2 (g) → pcl5 (g) an equilibrium mixture at 450 k contains ppcl3 = 0.348 atm, pcl2 = 0.441 atm, and ppcl5 = 10.24 atm. what is the value of kp at this temperature? phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: (g) + (g) (g) an equilibrium mixture at 450 k contains = 0.348 atm, = 0.441 atm, and = 10.24 atm. what is the value of kp at this temperature? 66.7 1.50 ⋅ 10−2 12.99 1.57 9.45

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlor...
Questions




Advanced Placement (AP), 05.05.2020 03:58

Mathematics, 05.05.2020 03:58








Mathematics, 05.05.2020 03:59

English, 05.05.2020 03:59

English, 05.05.2020 03:59



Mathematics, 05.05.2020 03:59

English, 05.05.2020 03:59

Social Studies, 05.05.2020 03:59

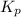 at this temperature is 66.7
at this temperature is 66.7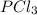 at equilibrium = 0.348 atm
at equilibrium = 0.348 atm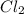 at equilibrium = 0.441 atm
at equilibrium = 0.441 atm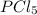 at equilibrium = 10.24 atm
at equilibrium = 10.24 atm