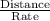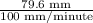In a gas chromatography experiment, a mixture of two compounds (a and b) was separated for analysis. a chart recorder that fed paper at a constant rate of 100 mm. per minute was used to record the chromatogram, and a mark was made on the chart at the time the sample was injected. the first peak on the chromatogram corresponds to compound a, and that peak was observed at a distance of 5.83 cm from the sample injection point, and it had a height of 3.52 cm and a width at half-height of 1.25 cm. the peak corresponding to b was observed at a distance of 7.96 cm from the injection point, and it had a height of 3.34 cm and a width at half-height of 1.06 cm. what was the retention time for compound b (in seconds)? (enter your answer as a number only.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3
You know the right answer?
In a gas chromatography experiment, a mixture of two compounds (a and b) was separated for analysis....
Questions

History, 26.07.2019 11:50

Mathematics, 26.07.2019 11:50



Physics, 26.07.2019 11:50




Physics, 26.07.2019 11:50


History, 26.07.2019 11:50



Mathematics, 26.07.2019 11:50

English, 26.07.2019 11:50

Mathematics, 26.07.2019 11:50

Mathematics, 26.07.2019 11:50

History, 26.07.2019 11:50

Social Studies, 26.07.2019 11:50

Mathematics, 26.07.2019 11:50