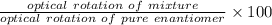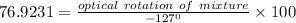Chemistry, 09.10.2019 22:20 hlannette7005
An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water and was found to have a specific rotation of −127°. if this solution were mixed with 500 ml of a solution containing 3 g of a racemic mixture of the compound, what would the specific rotation of the resulting mixture of the compound? what would be its optical purity?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water a...
Questions


Computers and Technology, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01




Computers and Technology, 20.10.2020 07:01

Social Studies, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01


Mathematics, 20.10.2020 07:01

Social Studies, 20.10.2020 07:01




Computers and Technology, 20.10.2020 07:01

History, 20.10.2020 07:01



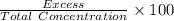 =
= 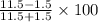 = 76.9231 %
= 76.9231 %