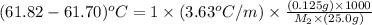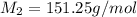Chemistry, 09.10.2019 18:00 Derienw6586
Benzyl acetate is one of the active components of oil of jasmine. if 0.125 g of the compound is added to 25.0g of chloroform (chcl 3 ), the boiling point of the solution is 61.82°c. what is the molar mass of benzyl acetate. (chloroform: kb = 3.63 °c/m; tb° 61.70°c)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
Benzyl acetate is one of the active components of oil of jasmine. if 0.125 g of the compound is adde...
Questions


Chemistry, 01.12.2021 03:30




Computers and Technology, 01.12.2021 03:30


Mathematics, 01.12.2021 03:30



Social Studies, 01.12.2021 03:30

World Languages, 01.12.2021 03:30



Chemistry, 01.12.2021 03:30

SAT, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30

Mathematics, 01.12.2021 03:30


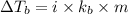
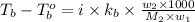
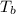 = boiling point of solution =
= boiling point of solution = 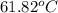
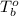 = boiling point of chloroform =
= boiling point of chloroform = 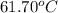
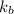 = boiling point constant of chloroform =
= boiling point constant of chloroform = 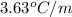
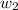 = mass of solute (Benzyl acetate) = 0.125 g
= mass of solute (Benzyl acetate) = 0.125 g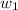 = mass of solvent (chloroform) = 25.0 g
= mass of solvent (chloroform) = 25.0 g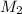 = molar mass of solute (Benzyl acetate) = ?
= molar mass of solute (Benzyl acetate) = ?