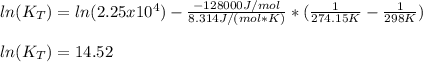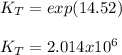Chemistry, 09.10.2019 16:30 parkerfreeze
Enter your answer in the provided box. the formation of methanol is important to the processing of new fuels. at 298.0 k, kp = 2.25 × 104 for the reaction co(g) + 2 h2(g) ⇌ ch3oh(l) if δh o rxn = −128 kj/ mol ch3oh, calculate kp at 1°c. × 10 -6 (enter your answer in scientific notation.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1


Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Enter your answer in the provided box. the formation of methanol is important to the processing of n...
Questions



Mathematics, 19.05.2021 23:00

Mathematics, 19.05.2021 23:00


Mathematics, 19.05.2021 23:00

Mathematics, 19.05.2021 23:00

Mathematics, 19.05.2021 23:00

Mathematics, 19.05.2021 23:00

Medicine, 19.05.2021 23:00



History, 19.05.2021 23:00


English, 19.05.2021 23:00

Mathematics, 19.05.2021 23:00


Mathematics, 19.05.2021 23:00


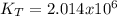
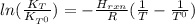
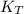 it means the equilibrium constant at 1 °C, we get:
it means the equilibrium constant at 1 °C, we get: