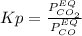Chemistry, 09.10.2019 05:30 minnahelhoor
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s) kp = 20 at 873 k if a reaction vessel at equilibrium contains solid ni, solid nio, 400 mm hg of co2, and 20 mm hg of co, doubling the amount of co(g) present will lead to the production of more solid nickel at 873 k. group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s)...
Questions




Mathematics, 31.03.2021 23:10

Mathematics, 31.03.2021 23:10

Social Studies, 31.03.2021 23:10

Biology, 31.03.2021 23:10










English, 31.03.2021 23:10

Mathematics, 31.03.2021 23:10

English, 31.03.2021 23:10