
Chemistry, 09.10.2019 03:30 fancycar14
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed to oxygen. the reaction is 2b5h9(l) + 12o2(g) ⟶ 5b2o3(s) + 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formation of b5h9 is 73.2 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Pentaborane-9, b5h9, is a colorless, highly reactive liquid that will burst into flame when exposed...
Questions

Mathematics, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50






Mathematics, 25.03.2021 18:50

Law, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50


Biology, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50

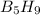 is -71.92 kJ
is -71.92 kJ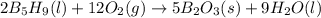
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0302/4270/72c39.png)
![\Delta H^o_{rxn}=[(5\times \Delta H^o_f_{(B_2O_3(s))})+(9\times \Delta H^o_f_{(H_2O(l))})]-[(2\times \Delta H^o_f_{(B_5H_9(l))})+(12\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0302/4270/e310e.png)
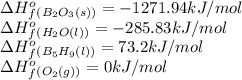
![\Delta H^o_{rxn}=[(5\times (1271.94))+(9\times (-285.83))]-[(2\times (73.2))+(12\times (0))]\\\\\Delta H^o_{rxn}=-9078.57kJ](/tpl/images/0302/4270/015ae.png)
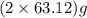 of
of 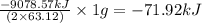 of energy.
of energy.

