
Chemistry, 09.10.2019 03:30 cranfordjacori
An aqueous solution of sodium sulfate is allowed to react with an aqueous solution of calciumnitrate. the complete ionic equation contains which of the following species (when balanced in stan-dard form)? a.2na+aq)b.2so42-(aq)c.3ca2+(aq)d. no3-(aq)e. k+(aq)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
An aqueous solution of sodium sulfate is allowed to react with an aqueous solution of calciumnitrate...
Questions






Computers and Technology, 21.12.2021 14:00



Computers and Technology, 21.12.2021 14:00

SAT, 21.12.2021 14:00

English, 21.12.2021 14:00

Mathematics, 21.12.2021 14:00




Computers and Technology, 21.12.2021 14:00



Mathematics, 21.12.2021 14:00

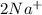
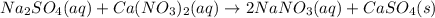
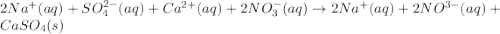
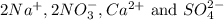 are the spectator ions.
are the spectator ions.

