
Chemistry, 09.10.2019 03:00 destiny465
H2so4 is an unusual diprotic acid in that the first dissociation is considered strong, but the second dissociation is weak. therefore, h2so4 only has ka2 = 1.2x10−2. consider an aqueous solution containing 0.010 m h2so4. calculate the concentration of so42− ions in this solution. calculate the concentration of hso4− ions in this solution. calculate the concentration of h3o+ ions the solution. enter each of your answers to two significant figures. do not use scientific notation.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1


You know the right answer?
H2so4 is an unusual diprotic acid in that the first dissociation is considered strong, but the secon...
Questions


Mathematics, 16.06.2020 13:57


Mathematics, 16.06.2020 13:57



Business, 16.06.2020 13:57

Geography, 16.06.2020 13:57





English, 16.06.2020 13:57



Biology, 16.06.2020 13:57

Mathematics, 16.06.2020 13:57


Mathematics, 16.06.2020 13:57

Mathematics, 16.06.2020 13:57

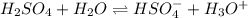
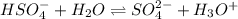
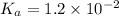 . Hence, formula for
. Hence, formula for 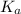 is as follows.
is as follows.![K_{a} = \frac{[SO^{2-}_{4}][H_{3}O^{+}]}{[HSO^{-}_{4}]}](/tpl/images/0302/2941/4c298.png)
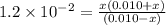
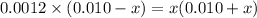
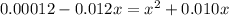
![[SO^{2-}_{4}]](/tpl/images/0302/2941/a4a68.png) = x,
= x, ![[HSO^{-}_{4}]](/tpl/images/0302/2941/b5b89.png) = 0.010 - x
= 0.010 - x![[H_{3}O^{+}]](/tpl/images/0302/2941/71b58.png) = 0.010 + x
= 0.010 + x

