
Chemistry, 09.10.2019 01:00 jtaylor4061
The reaction of silver metal and dilute nitric acid proceeds according to the equation above. if 0.10 mole of powdered silver is added to 10.0 milliliters of 6.0 molar nitric acid, the number of moles of no gas that can be formed is
a) 0.015 mole
b) 0.020 mole
c) 0.030 mole
d) 0.045 mole
e) 0.090 mole

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
You know the right answer?
The reaction of silver metal and dilute nitric acid proceeds according to the equation above. if 0.1...
Questions


Biology, 15.09.2021 23:20

History, 15.09.2021 23:20

Social Studies, 15.09.2021 23:20

English, 15.09.2021 23:20

English, 15.09.2021 23:20

Mathematics, 15.09.2021 23:20

Mathematics, 15.09.2021 23:20

Mathematics, 15.09.2021 23:20


Mathematics, 15.09.2021 23:20



English, 15.09.2021 23:20



Mathematics, 15.09.2021 23:20

Chemistry, 15.09.2021 23:20

Social Studies, 15.09.2021 23:20

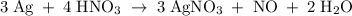
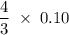 moles nitric acid
moles nitric acid

