
Chemistry, 09.10.2019 00:00 school4life110
The ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. in the first step of the ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water. what is the maximum mass of h2o that can be produced by combining 90.1 g of each reactant? 4nh3(g)+5o2(g)⟶4no(g)+6h2o(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 23.06.2019 13:30
Asap what is the temperature when the volume is 700 ml? a 500 k b 200 k c 600 k d 700 k
Answers: 1

You know the right answer?
The ostwald process is used commercially to produce nitric acid, which is, in turn, used in many mod...
Questions

History, 02.12.2020 22:40

Mathematics, 02.12.2020 22:40



Mathematics, 02.12.2020 22:40

Mathematics, 02.12.2020 22:50


Mathematics, 02.12.2020 22:50

Mathematics, 02.12.2020 22:50

Mathematics, 02.12.2020 22:50

Mathematics, 02.12.2020 22:50


Business, 02.12.2020 22:50

Computers and Technology, 02.12.2020 22:50





Mathematics, 02.12.2020 22:50

Chemistry, 02.12.2020 22:50

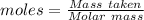
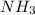
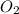
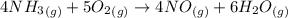
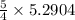 mole of
mole of 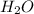
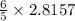 moles of
moles of 

