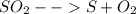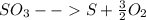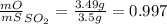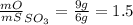Chemistry, 08.10.2019 05:30 aboatright7410
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decomposed the sulfur dioxide produced 3.49g oxygen and 3.50g sulfur, while the sulfur trioxide produced 9.00g oxygen and 6.00g sulfur.
a) calculate the mass of oxygen per gram of sulfur for sulfur dioxide.
b) calculate the mass of oxygen per gram of sulfur for sulfur trioxide.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decompose...
Questions


Physics, 23.02.2022 14:00

Geography, 23.02.2022 14:00

SAT, 23.02.2022 14:00





SAT, 23.02.2022 14:00

Spanish, 23.02.2022 14:00

Health, 23.02.2022 14:00






Business, 23.02.2022 14:00