
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to remove h2s is to treat the water with chlorine, in which case the following reaction occurs: h2s(aq)+cl2(aq)→s(s)+2h+(aq)+2cl−(a q) the rate of this reaction is first order in each reactant. the rate constant for the disappearance of h2s at 28 ∘c is 3.5×10−2 m−1s−1.if at a given time the concentration of h2s is 2.0×10-4 m and that of cl2 is 2.8×10-2 m , what is the rate of formation of cl?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to r...
Questions

Mathematics, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01

English, 20.09.2020 19:01


Geography, 20.09.2020 19:01

Biology, 20.09.2020 19:01

Social Studies, 20.09.2020 19:01

Chemistry, 20.09.2020 19:01


English, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01

Spanish, 20.09.2020 19:01



Physics, 20.09.2020 19:01

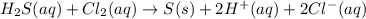
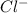 will be twice the rate of disappearance of
will be twice the rate of disappearance of 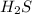 .
.![k[H_{2}S][Cl_{2}]](/tpl/images/0299/3822/2cbd6.png)
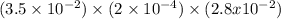
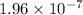 M/s
M/s
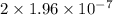
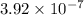 M/s
M/s

