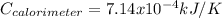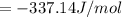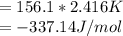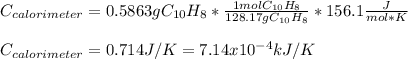When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter constant, a temperature rise of 2.862 k was measured near 298k. under similar conditions, a temperature rise of 2.416 k was measured when a 0.5863 g naphthalene sample was burned. determine the calorimeter constant (in units of kj/k) and the standard enthalpy of combustion for naphthalene at 298k. if we make the assumption that ∆h ≈∆u, is the ∆chº value obtained from this experim

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1
You know the right answer?
When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter co...
Questions




Mathematics, 07.07.2019 09:00

Mathematics, 07.07.2019 09:00



Mathematics, 07.07.2019 09:00


Mathematics, 07.07.2019 09:00

History, 07.07.2019 09:00

History, 07.07.2019 09:00


Mathematics, 07.07.2019 09:00

Mathematics, 07.07.2019 09:00


History, 07.07.2019 09:00

Health, 07.07.2019 09:00


Biology, 07.07.2019 09:00