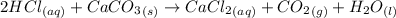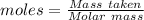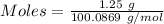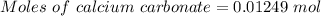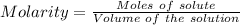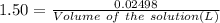Chemistry, 08.10.2019 05:10 pressure772
Calcium carbonate is added to separate solutions of hydrochloric acid and ethanoic acid of the same concentration. state one similarity and one difference in the observations you could make.
(i) write an equation for the reaction between hydrochloric acid and calcium carbonate.
(ii) determine the volume of 1.50 mol/dm–3 hydrochloric acid that would react with exactly 1.25 g of calcium carbonate.
(iii) calculate the volume of carbon dioxide, measured at 273 k and 1.01×105 pa, which would be produced when 1.25 g of calcium carbonate reacts completely with the hydrochloric acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1


Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
Calcium carbonate is added to separate solutions of hydrochloric acid and ethanoic acid of the same...
Questions

Computers and Technology, 03.05.2021 15:10


Computers and Technology, 03.05.2021 15:10



Health, 03.05.2021 15:10

Social Studies, 03.05.2021 15:10

Business, 03.05.2021 15:10





Mathematics, 03.05.2021 15:10

English, 03.05.2021 15:10