
Chemistry, 08.10.2019 04:30 bonnysvalentine
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major isotope is 24mg, natural abundance 78.99%, relative atomic mass 23.98504. the next most abundant isotope is 26mg, relative atomic mass 25.98259. the third most abundant isotope is 25mg whose natural abun- dance is in the ratio of 0.9083 to that of 26mg. find the relative atomic mass of 25mg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major...
Questions








History, 15.01.2020 05:31

Spanish, 15.01.2020 05:31

English, 15.01.2020 05:31

English, 15.01.2020 05:31

Mathematics, 15.01.2020 05:31

Spanish, 15.01.2020 05:31


Computers and Technology, 15.01.2020 05:31


Health, 15.01.2020 05:31


Mathematics, 15.01.2020 05:31

Chemistry, 15.01.2020 05:31

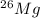 , then 0.9083x represents % for
, then 0.9083x represents % for 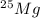 .
.

