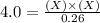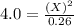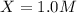Chemistry, 08.10.2019 01:00 hamadehassan
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l container and allowed to come to equilibrium, and the equilibrium concentration of pcl5(g) is 0.26 m, what is the equilibrium concentration of pcl3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 23.06.2019 15:00
The specific heat of a certain type of cooking oil is 1.75 cal/ (g c) how much heat energy is needed to raise the temperature of 2.67 kg of this oil from 23 c to 191 c
Answers: 1
You know the right answer?
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l...
Questions

Health, 18.11.2019 10:31


Biology, 18.11.2019 10:31

Social Studies, 18.11.2019 10:31


Chemistry, 18.11.2019 10:31



Spanish, 18.11.2019 10:31

English, 18.11.2019 10:31

History, 18.11.2019 10:31

Physics, 18.11.2019 10:31




Social Studies, 18.11.2019 10:31

Mathematics, 18.11.2019 10:31



 is, 1.0 M
is, 1.0 M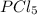 = 0.26 M
= 0.26 M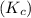 = 4.0
= 4.0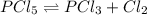
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0298/6790/73fe0.png)
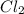 are equal.
are equal.