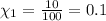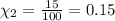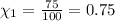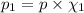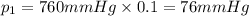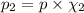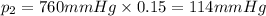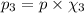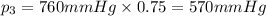Imagine a planet that has a similar atmospheric pressure to earth (760 mmhg) but different concentrations of gases in its atmosphere. on this hypothetical planet, oxygen makes up 10% of the atmosphere, carbon dioxide makes up 15%, and nitrogen makes up the remaining 75%. based on this information, what is the partial pressure of oxygen on this new planet?
646 mmhg
114 mmhg
160 mmhg
76 mmhg
571 mmhg

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
Imagine a planet that has a similar atmospheric pressure to earth (760 mmhg) but different concentra...
Questions





Chemistry, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30

Law, 18.03.2021 03:30

Chemistry, 18.03.2021 03:30


Biology, 18.03.2021 03:30





Mathematics, 18.03.2021 03:30



Mathematics, 18.03.2021 03:30