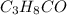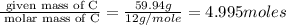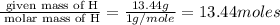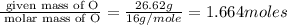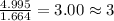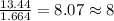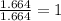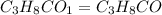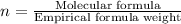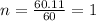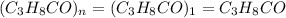Chemistry, 07.10.2019 22:30 MichaelBoolin87241
Acompound is found to contain 59.94 % carbon , 13.44 % hydrogen , and 26.62 % oxygen by mass.
(1) the empirical formula for this compound is .
(2) the molecular weight for this compound is 60.11 amu. the molecular formula for this compound is

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Acompound is found to contain 59.94 % carbon , 13.44 % hydrogen , and 26.62 % oxygen by mass.
...
...
Questions


Chemistry, 03.10.2021 21:10

English, 03.10.2021 21:10

English, 03.10.2021 21:20


History, 03.10.2021 21:20

Mathematics, 03.10.2021 21:20

Physics, 03.10.2021 21:20


Chemistry, 03.10.2021 21:20


Arts, 03.10.2021 21:20

Mathematics, 03.10.2021 21:20

Mathematics, 03.10.2021 21:20



Arts, 03.10.2021 21:20



Mathematics, 03.10.2021 21:20