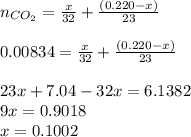An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5oh ). a 0.220-g m g sample of the liquid is burned in an excess of o2(g) m o_2(g) and yields 0.367g g co2(g) m co_2(g) (carbon dioxide). set up two algebraic equations, one expressing the mass of carbon dioxide produced in terms of each reagent and the other expressing the mass of sample burned in terms of each reagent.
what is the mass of methyl alcohol (ch3oh m ch_3oh) in the sample?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

You know the right answer?
An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5...
Questions

Mathematics, 03.12.2020 20:50

Mathematics, 03.12.2020 20:50



Mathematics, 03.12.2020 20:50

English, 03.12.2020 20:50


Mathematics, 03.12.2020 20:50

Mathematics, 03.12.2020 20:50

Mathematics, 03.12.2020 20:50

Health, 03.12.2020 20:50


English, 03.12.2020 20:50

Mathematics, 03.12.2020 20:50





Mathematics, 03.12.2020 20:50

Mathematics, 03.12.2020 20:50

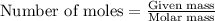 .....(1)
.....(1)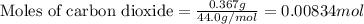
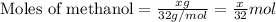
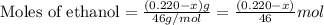
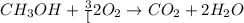
![\frac{x}{32} moles of methanol will produce = [tex]\frac{1}{1}\times \frac{x}{32}=\frac{x}{32}](/tpl/images/0297/8500/66d68.png) moles of carbon dioxide
moles of carbon dioxide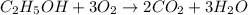
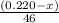 moles of ethanol will produce =
moles of ethanol will produce = 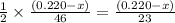 moles of carbon dioxide
moles of carbon dioxide