
Chemistry, 07.10.2019 18:30 oliviaclerk5
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable binary ionic compound. is he correct? why or why not?
a hector is incorrect. the two ions have opposite charges, so they will bond together in a binary ionic compound.
b hector is incorrect. the two ions have more than eighteen protons, so they will bond together in a binary ionic compound.
c hector is correct. the sum of the charges of the two ions must be zero in order for them to form a stable ionic compound.
d hector is correct. the sum of the protons of the two ions must be divisible by eight in order for them to form a stable ionic compound.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3


Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable...
Questions

Mathematics, 03.05.2020 13:40









Mathematics, 03.05.2020 13:40

Mathematics, 03.05.2020 13:40

Mathematics, 03.05.2020 13:40


Mathematics, 03.05.2020 13:40


History, 03.05.2020 13:40

Mathematics, 03.05.2020 13:40

Mathematics, 03.05.2020 13:40

History, 03.05.2020 13:40

![[Na]=1s^22s^22p^63s^1](/tpl/images/0297/6892/4ee3b.png)
![[O]=1s^22s^22p^4](/tpl/images/0297/6892/336ff.png)
![[O^{2-}]=1s^22s^22p^6](/tpl/images/0297/6892/c797e.png)
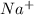 cation and oxide
cation and oxide 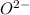 is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral 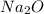 .
.

