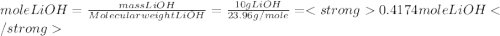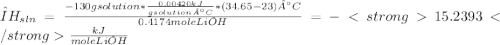A10.0 g sample of lioh is dissolved in 120.00 g of water at 23.0â°c in a coffee-cup calorimeter with a heat capacity of 100 j/â°c. the heat capacity of the solution was 4.20 j/gâ°c, and final temperature was 34.65â°c. based on these measurements, what is the enthalpy of dissolution of lioh in kj/mol?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
A10.0 g sample of lioh is dissolved in 120.00 g of water at 23.0â°c in a coffee-cup calorimeter with...
Questions


History, 25.09.2019 01:00


Health, 25.09.2019 01:00



Social Studies, 25.09.2019 01:00


Health, 25.09.2019 01:00




Mathematics, 25.09.2019 01:00



Computers and Technology, 25.09.2019 01:00



Biology, 25.09.2019 01:00

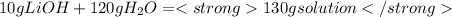
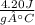
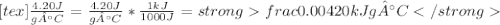 " alt="\frac{4.20 J}{g°C}=\frac{4.20 J}{g°C}*\frac{1 kJ}{1000 J}=\frac{0.00420 kJ}{g°C}" />" />
" alt="\frac{4.20 J}{g°C}=\frac{4.20 J}{g°C}*\frac{1 kJ}{1000 J}=\frac{0.00420 kJ}{g°C}" />" />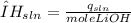 Equation 1
Equation 1
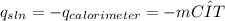 Equation 2
Equation 2 ![mole of LiOH=\frac{mas of LiOH}{Molecular weight of LiOH} Equation 3 Where:[tex]ΔH_{sln}](/tpl/images/0297/3554/4ff05.png) = enthalpy of dissolution per mole of LiOH (kJ/mole).
= enthalpy of dissolution per mole of LiOH (kJ/mole).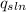 = heat released by dissolution (kJ).
= heat released by dissolution (kJ).  =heat absorbed by the solution in calorimeter (kJ)
.
=heat absorbed by the solution in calorimeter (kJ)
.