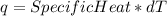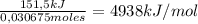Chemistry, 07.10.2019 16:10 dillondelellis2006
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose, c12h22o11, was burned in a bomb calorimeter with a heat capacity of 7.50 kj/oc (including its water). the temperature inside the calorimeter was found to increase by 20.2 oc. based on this information, what is the heat of this reaction per mole of sucrose?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3


Chemistry, 23.06.2019 11:40
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2
You know the right answer?
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose...
Questions

Mathematics, 17.12.2020 23:50


Mathematics, 17.12.2020 23:50

Advanced Placement (AP), 17.12.2020 23:50

Mathematics, 17.12.2020 23:50





English, 17.12.2020 23:50

Chemistry, 17.12.2020 23:50

Spanish, 17.12.2020 23:50


Chemistry, 17.12.2020 23:50



Mathematics, 17.12.2020 23:50



Health, 17.12.2020 23:50