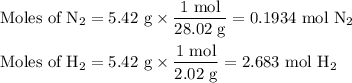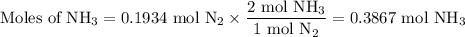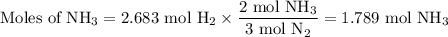N2(g) + 3 h2(g) > 2 hn3(g) (blanced)
if 5.42 g of nitrogen are reacted with 5.42 g o...

Chemistry, 07.10.2019 00:10 ujusdied5176
N2(g) + 3 h2(g) > 2 hn3(g) (blanced)
if 5.42 g of nitrogen are reacted with 5.42 g of hydrogen gas, which of the reactants is the limiting reactant?
molar mass of n2 = 28.02 g/mol
molar mass of h2 = 2.02 g/mol
molar mass of nh3 = 17.04 g/mol

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Questions



Mathematics, 20.09.2020 09:01

Geography, 20.09.2020 09:01


English, 20.09.2020 09:01

History, 20.09.2020 09:01


Health, 20.09.2020 09:01


History, 20.09.2020 09:01

Arts, 20.09.2020 09:01

Social Studies, 20.09.2020 09:01

Physics, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01



History, 20.09.2020 09:01