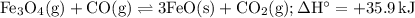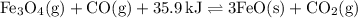Consider the following reaction at equilibrium. what effect will increasing the
temperature ha...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Questions

English, 27.12.2021 01:00

Mathematics, 27.12.2021 01:00

English, 27.12.2021 01:00

English, 27.12.2021 01:00


Mathematics, 27.12.2021 01:00



English, 27.12.2021 01:00


Biology, 27.12.2021 01:00

Arts, 27.12.2021 01:00


English, 27.12.2021 01:00


Mathematics, 27.12.2021 01:00

Mathematics, 27.12.2021 01:00


Mathematics, 27.12.2021 01:00