
Chemistry, 06.10.2019 17:20 danielahalesp87vj0
Stoichiometry questiom ?
c6h12o6(s) +6o2(g) -> 6co2(g) + 6h2o(l)
the human body needs at least 1.03 x 10-2 mol o2 every minute. if all of this oxygen is used for the
cellular respiration reaction that breaks down glucose, how many grams of glucose does the human body
consume each minute?
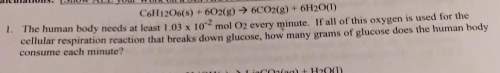

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Stoichiometry questiom ?
c6h12o6(s) +6o2(g) -> 6co2(g) + 6h2o(l)
the hum...
c6h12o6(s) +6o2(g) -> 6co2(g) + 6h2o(l)
the hum...
Questions


Geography, 13.07.2020 22:01

Mathematics, 13.07.2020 22:01





Mathematics, 13.07.2020 22:01

Physics, 13.07.2020 22:01




Mathematics, 13.07.2020 22:01






Mathematics, 13.07.2020 22:01

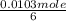 ;
;


