
Chemistry, 06.10.2019 19:30 opgbadwolf5
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compound with antioxidant properties. a healthy adult’s daily requirement of vitamin c is 70-90 mg. a sweet lime contains 2.82×10−4 mol of ascorbic acid.
to determine whether the ascorbic acid in a sweet lime meets the daily requirement, calculate the mass of ascorbic acid in 2.82×10−4 mol of ascorbic acid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

You know the right answer?
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compo...
Questions

Mathematics, 23.04.2020 08:20

History, 23.04.2020 08:20

Mathematics, 23.04.2020 08:20

Mathematics, 23.04.2020 08:20


Mathematics, 23.04.2020 08:20


Spanish, 23.04.2020 08:20


Mathematics, 23.04.2020 08:21


Biology, 23.04.2020 08:21


Mathematics, 23.04.2020 08:21



Mathematics, 23.04.2020 08:21

History, 23.04.2020 08:21

Chemistry, 23.04.2020 08:21

English, 23.04.2020 08:21

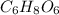 ) contains 6, C atoms 8, H atoms and 6, O atoms .
) contains 6, C atoms 8, H atoms and 6, O atoms .
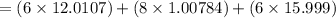
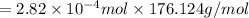
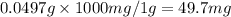 VITAMIN C
VITAMIN C

