
Chemistry, 06.10.2019 15:00 AnonymousLizard52303
Assist ! 40 points
a mercury lamp emits radiation with a frequency of 6.83 x 1014 hz. what is the wavelength of this radiation?
fill in the blanks as you answer the question. remember to include units. (if you need to write a superscript, put a ^ before the number to be superscripted. for example, x2 would be written as x^2.)
step 2: what is the speed of light to four significant figures?
step 3: what is the frequency of the light from the mercury lamp?
step 4: what is the wavelength of the light from the mercury lamp? blank space 3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Assist ! 40 points
a mercury lamp emits radiation with a frequency of 6.83 x 1014 hz. w...
a mercury lamp emits radiation with a frequency of 6.83 x 1014 hz. w...
Questions

History, 29.01.2021 01:50



Mathematics, 29.01.2021 01:50

English, 29.01.2021 01:50

Social Studies, 29.01.2021 01:50


Physics, 29.01.2021 01:50


History, 29.01.2021 01:50


Mathematics, 29.01.2021 01:50

Mathematics, 29.01.2021 01:50

History, 29.01.2021 01:50


Health, 29.01.2021 01:50



Mathematics, 29.01.2021 01:50

Mathematics, 29.01.2021 01:50

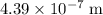 .
.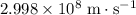 .
.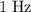 means one oscillation per second. The frequency of this particular wave is
means one oscillation per second. The frequency of this particular wave is 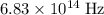 . In other words, there are
. In other words, there are  oscillations in each second.
oscillations in each second. 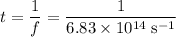 .
.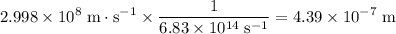 .
.

