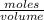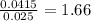Chemistry, 06.10.2019 10:02 Smartpotato9555
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1678 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrating it with the standard solution. the endpoint was reached after the addition of 19.88 ml of the standard solution. what is the concentration of the original sulfuric acid solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Achemist needs to determine the concentration of a sulfuric acid solution by titration with a standa...
Questions




Social Studies, 06.11.2021 07:20


English, 06.11.2021 07:20

Mathematics, 06.11.2021 07:30





English, 06.11.2021 07:30


Chemistry, 06.11.2021 07:30





Mathematics, 06.11.2021 07:30

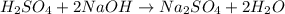
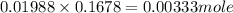
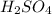 in 10 mL of diluted solution = 1/2 x moles of NaOH
in 10 mL of diluted solution = 1/2 x moles of NaOH
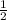 x 0.00333 = 0.00166 mol
x 0.00333 = 0.00166 mol
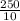 x 0.00166 = 0.0415 mol
x 0.00166 = 0.0415 mol