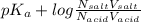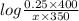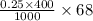Chemistry, 05.10.2019 04:20 Sumitco9578
Calculate how to prepare 750 ml of 0.25 m sodium formate buffer at ph 4. use your textbook to determine the molecular weight and pka of the acid and base. calculate the grams of sodium formate and number of milliliters of formic acid required. then using this stock solution, calculate and describe how you would prepare 100 ml of a 10 mm formate buffer, ph 3.5. by the way, what is the molarity of formic acid?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
Calculate how to prepare 750 ml of 0.25 m sodium formate buffer at ph 4. use your textbook to determ...
Questions


Mathematics, 25.12.2019 18:31

Mathematics, 25.12.2019 18:31



Mathematics, 25.12.2019 18:31



Mathematics, 25.12.2019 18:31

English, 25.12.2019 18:31



Mathematics, 25.12.2019 18:31


Mathematics, 25.12.2019 18:31


Mathematics, 25.12.2019 18:31


Mathematics, 25.12.2019 18:31

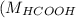 = 46 g/mol
= 46 g/mol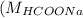 = 68 g/mol
= 68 g/mol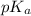 of HCOOH = 3.75
of HCOOH = 3.75![[HCOO^{-}]](/tpl/images/0288/1557/bee9e.png) = [HCOOH] + [HCOONa]
= [HCOOH] + [HCOONa]