
Chemistry, 05.10.2019 04:10 shels10tay
Be sure to answer all parts. write the balanced equations corresponding to the following rate expressions: a) rate = − 1 3 δ[ch4] δt = − 1 2 δ[h2o] δt = − δ[co2] δt = 1 4 δ[ch3oh] δt (click in the answer box to activate the palette. do not include states of matter.) b) rate = − 1 2 δ[n2o5] δt = 1 2 δ[n2] δt = 1 5 δ[o2] δt (click in the answer box to activate the palette. do not include states of matter.) c) rate = − 1 2 δ[h2] δt = − 1 2 δ[co2] δt = − δ[o2] δt = 1 2 δ[h2co3] δt (click in the answer box to activate the palette. do not include states of matter.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
Be sure to answer all parts. write the balanced equations corresponding to the following rate expres...
Questions

Mathematics, 16.12.2020 01:30





Mathematics, 16.12.2020 01:30


Mathematics, 16.12.2020 01:30

Mathematics, 16.12.2020 01:30

Mathematics, 16.12.2020 01:30


Chemistry, 16.12.2020 01:30

Mathematics, 16.12.2020 01:30

Mathematics, 16.12.2020 01:30

Mathematics, 16.12.2020 01:30

Physics, 16.12.2020 01:30

History, 16.12.2020 01:30


Mathematics, 16.12.2020 01:30

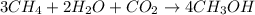
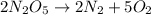
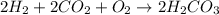
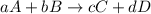
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0288/1459/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0288/1459/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0288/1459/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0288/1459/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0288/1459/d4b94.png)
![Rate=-\frac{1}{3}\frac{d[CH_4]}{dt}=-\frac{1}{2}\frac{d[H_2O]}{dt}=-\frac{d[CO_2]}{dt}=+\frac{1}{4}\frac{d[CH_3OH]}{dt}](/tpl/images/0288/1459/a1c90.png)
![Rate=-\frac{1}{2}\frac{d[N_2O_5]}{dt}=+\frac{1}{2}\frac{d[N_2]}{dt}=+\frac{1}{5}\frac{d[O_2]}{dt}](/tpl/images/0288/1459/697db.png)
![Rate=-\frac{1}{2}\frac{d[H_2]}{dt}=-\frac{1}{2}\frac{d[CO_2]}{dt}=-\frac{d[O_2]}{dt}=+\frac{1}{2}\frac{d[H_2CO_3]}{dt}](/tpl/images/0288/1459/13070.png)


