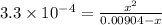The active ingredient in aspirin is acetylsalicylic acid (hc9h7o4), a monoprotic acid with a ka of 3.3×10−4 at 25 ∘c . you may want to reference (pages 680 - 690) section 16.6 while completing this problem. what is the ph of a solution obtained by dissolving two extra-strength aspirin tablets, containing 440 mg of acetylsalicylic acid each, in 270 ml of water? express your answer to two decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3
You know the right answer?
The active ingredient in aspirin is acetylsalicylic acid (hc9h7o4), a monoprotic acid with a ka of 3...
Questions

Physics, 19.07.2019 11:00

Mathematics, 19.07.2019 11:00

History, 19.07.2019 11:00

Mathematics, 19.07.2019 11:00

Mathematics, 19.07.2019 11:00


Mathematics, 19.07.2019 11:00



Mathematics, 19.07.2019 11:00







Chemistry, 19.07.2019 11:00



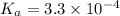
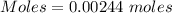
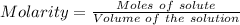
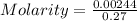
![K_{a}=\frac {\left [ H^{+} \right ]\left [ {C_9H_7O_4}^- \right ]}{[HC_9H_7O_4]}](/tpl/images/0288/1433/becfb.png)