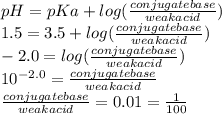Chemistry, 05.10.2019 04:10 meababy2009ow9ewa
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question. aspirin is a weak acid with a pka of 3.5 that is absorbed more effectively in the stomach than the small intestine. the ph of your stomach is around 1.5 and the ph of your small intestine is approximately 6.0. is aspirin absorbed more readily when it is protonated or deprotonated? what is the approximate ratio of conjugate base to acid when it is absorbed more readily?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Use the henderson-hasselbalch equation and your knowledge of ionization to you answer this question...
Questions
















Chemistry, 26.07.2019 14:30



English, 26.07.2019 14:30