Consider the following reaction between mercury(ii) chloride and oxalate ion.
2 hgcl2(aq...

Chemistry, 06.10.2019 10:00 hannahmorgret7811
Consider the following reaction between mercury(ii) chloride and oxalate ion.
2 hgcl2(aq) + c2o42-(aq) 2 cl -(aq) + 2 co2(g) + hg2cl2(s)
the initial rate of this reaction was determined for several concentrations of hgcl2 and c2o42-, and the following rate data were obtained for the rate of disappearance of c2o42-.
experiment [hgcl2] (m) [c2o42-] (m) rate (m/s)
1 0.164 0.15 3.2x10^-5
2 0.164 0.45 2.9x10^-4
3 0.082 0.45 1.4x10^-4
4 0.246 0.15 4.8x10^-5
what is the rate law for this reaction?
(a) -k[hgcl2][c2o4-2]2-
(b) -k[hgcl2]2[c2o4-2]
(c) -k[hgcl2]2[c2o4-2]1/2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

You know the right answer?
Questions




















![\text{Rate}=k[HgCl_2][C_2O_4^{2-}]^2](/tpl/images/0293/1267/42a62.png)

![\text{Rate}=k[HgCl_2]^a[C_2O_4^{2-}]^b](/tpl/images/0293/1267/af610.png)
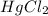
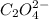
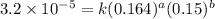 ....(1)
....(1)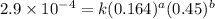 ....(2)
....(2)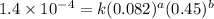 ....(3)
....(3)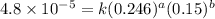 ....(4)
....(4) 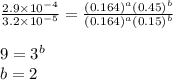
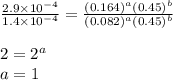
![\text{Rate}=k[HgCl_2]^1[C_2O_4^{2-}]^2](/tpl/images/0293/1267/956be.png)


