
Chemistry, 05.10.2019 02:10 Whitehouse9
Write the chemical equation and the ka expression for the acid dissociation of each of the following acids in aqueous solution. first show the reaction with h+(aq) as a product and then with the hydronium ion. part a c6h5cooh. show the reaction with h+(aq) as a product. express your answer as a chemical equation. identify all of the phases in your answer. nothing request answer part b write the ka expression for the acid dissociation from the previous part. write the expression for the acid dissociation from the previous part. 1[h+][c6h5coo−] [c6h5cooh][h+][c6h5coo−] [h+][c6h5coo−][c6h5cooh] [h+][c6h5coo−]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Write the chemical equation and the ka expression for the acid dissociation of each of the following...
Questions

Mathematics, 01.02.2020 00:58





Chemistry, 01.02.2020 00:58



Mathematics, 01.02.2020 00:58







English, 01.02.2020 00:58

Mathematics, 01.02.2020 00:58

Chemistry, 01.02.2020 00:58


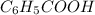
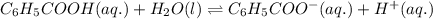
![K_a=\frac{[C_6H_5COO^-][H^+]}{[C_6H_5COOH]}](/tpl/images/0287/8245/438a5.png)


