
Chemistry, 06.10.2019 09:02 oliviaschmitt0
The blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of cr3+(aq) and carbon dioxide. the reaction can be monitored because the dichromate ion (cr2o72−) is orange in solution, and the cr3+ ion is green. the unbalanced redox equation is the following. cr2o72−(aq) + c2h5oh(aq) → cr3+(aq) + co2(g) if 31.91 ml of 0.0613 m potassium dichromate solution is required to titrate 33.8 g of blood plasma, determine the mass percent of alcohol in the blood.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

You know the right answer?
The blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with an aci...
Questions


English, 14.09.2021 14:00

Biology, 14.09.2021 14:00





World Languages, 14.09.2021 14:00


Mathematics, 14.09.2021 14:00





Mathematics, 14.09.2021 14:00

English, 14.09.2021 14:00

Biology, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00

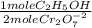 = 9,780x10⁻⁴ moles of C₂H₅OH
= 9,780x10⁻⁴ moles of C₂H₅OH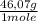 = 0,04506 g of C₂H₅OH
= 0,04506 g of C₂H₅OH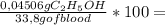 0,1333% of alcohol in the blood
0,1333% of alcohol in the blood

